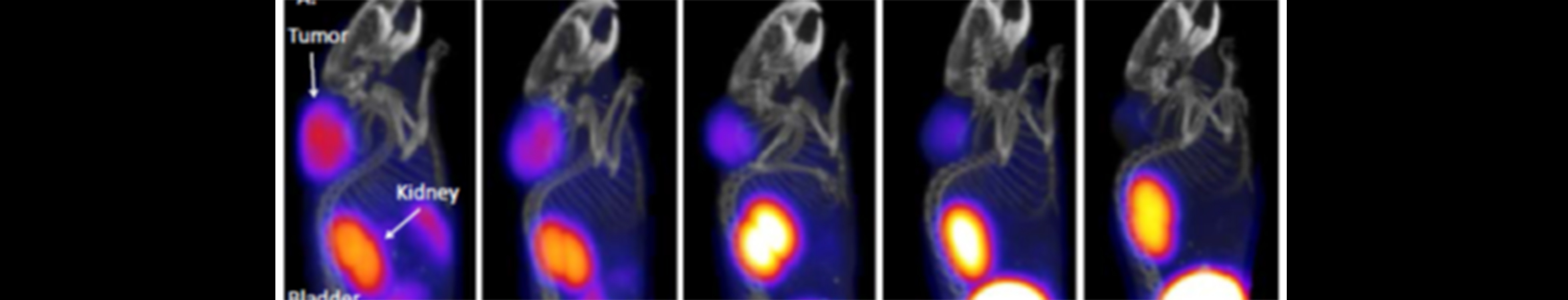
Radiochemistry
Overview
Our radiochemistry team has successfully radiolabeled hundreds of biological agents and small molecules and submitted over 80 INDs for clinical use of novel radiotracers. This wide range of chemical entities has included CNS-penetrant small molecules, monoclonal antibodies, peptides, intrathecally administered molecules, virus and live cell labeling, and heart and blood targeting agents.
In addition to radiochemistry services, we also offer ligand design capabilities and medicinal chemistry support for optimization and synthesis of new ligands. This includes an in-house organic synthesis lab and partnered CROs for outsourcing DMPK analysis. In addition to in vitro screening, we also perform in silico virtual screening of existing compound libraries to aid in your ligand selection processes.
Capabilities
Across our multiple sites, we offer the following services for radiotracer support:
- Radiohalogenation and radiometallation
- Manufacturing for clinical use according to GMP, USP 823, USP 825, and CFR part 212
- Deep expertise in automated synthesis module, including GE, Eckert and Zeigler, and ORA systems
- Wide range of ligands, ranging from low molecular weight to proteins and biologics.
- Ga-68 generator on site
- Siemens Cyclone cyclotrons for C-11 and F-18 production
- Rapid method transfers between sites
- Shipping of manufactured tracers to off-site imaging facilities
- Outsourcing tritiation of ligands
In addition to manufacturing capabilities, we provide the following associated quality control services:
- HPLC methodology including RP, SE, IE and UPLC
- In-house development of bioactivity and immunoreactivity assays
- Full metabolite analysis (blood, urine, and brain)
- Cell binding, tissue binding and cell internalization assay method development
- CMP panel/urine analysis for monitoring metabolic health
- UV/Vis spectrophotometer
- GC for residual solvent analysis
- SDS-page electrophoresis
- Gamma counters
- Dose calibrator and multi-channel analyzers
- Radio-TLC scanners
- Audio radiography
Isotopes/Modalities
Invicro has experience working on projects with common SPECT/PET isotopes. We also support conjugation with fluorophores as well as ‘cold’ radiolabeling with non-radioactive elements.
Common SPECT Isotopes
| Isotope | Half-Life |
| 99mTc | 6.01 hrs |
| 123I | 13.2 hrs |
| 111In | 67.3 hrs |
| 201Tl | 73.0 hrs |
| 177Lu | 159.5 hrs |
| 131I | 192.6 hrs |
Common PET Isotopes
| Isotope | Half-Life |
| 13N | 10 mins |
| 11C | 20.3 mins |
| 68Ga | 67.7 mins |
| 18F | 67.7 mins |
| 64Cu | 12.7 hrs |
| 89Zr | 78.4 hrs |
| 124I | 4.2 days |
| 52Mn | 5.6 days |
Other Available Isotopes
| Isotope | Half-Life |
| 213Bi | 45.6 mins |
| 90Y | 64.1 hrs |
| 225Ac | 10.0 days |
| 32P | 14.3 days |
| 3H | 12.3 yrs |
| 14C | 5730 yrs |
Medicinal Chemistry
Invicro offers comprehensive capabilities in small molecule ligand development, by consulting on the use of sponsor-derived ligand libraries or manufacturing custom precursors and standards for preclinical applications. Our in-house expertise in ligand selection criteria and screening helps drive more informed go/no-go decisions for early-phase candidates. Additionally, we provide custom GMP precursor manufacturing through our trusted contracted partners.
Technology Transfer Services
In addition to our in-house radiochemistry services, we also provide oversight of clinical manufacturing from external sites. This includes production and shipment from our New Haven facility to locations across the US, and from our London facility to sites across the UK.
We also offer access to and setup of a worldwide network of radiotracers manufacturers. This is achieved by bringing the chemistry manufacturing to one of our GMP sites before sending our technology transfer teams to the sponsor-identified site to set up the manufacturing process.
We also have experience working with external radiochemistry manufacturers to optimize and bring new tracers with IND applications and NDA for commercial filing.
Thought Leadership Content
Case Studies
Development of Novel Adenosine 2a Receptor Ligands
Radiolabeling of a Virus for Intrathecal Injection and Biodistribution Assessment
Radiolabeling of a DOTA Conjugate Antibody for Theranostic Applications
Webinars
Translating Radiolabeled Biologics – Strategies for Successful IND Submission
Development of Novel PET Radioligands for PET Imaging Applications
Chemistry External Manufacturing and Network Management
