
PSMA-PET
Advancing the PSMA-PET Standard of Care
OVERVIEW
Prostate cancer is the most common type of cancer (aside from skin) in American men with 13 out of every 100 being diagnosed with the disease during their lifetime.1,2Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is expressed in high levels in prostate cancer cells. Due to its location on the cell surface, PSMA is a popular target for imaging to identify the presence of prostate cancer and to deliver drugs directly to the cancerous cells3.
A PSMA-PET scan is a powerful molecular imaging technique that can accurately localize prostate cancer and assess the level of a tumor’s PSMA expression. There are currently two USFDA and one EMA-approved PSMA-PET agents that are widely used in both clinic and trials. In early 2022, the USFDA approved the first targeted radioligand therapy, 177Lu-PSMA-61, for use in men with metastatic prostate cancer whose tumors overexpress the PSMA protein4.
Invicro is a full-service CRO providing industry-leading PSMA-PET solutions from radiochemistry development to preclinical and Phase 0-III clinical trials. We support researchers that develop PSMA-targeted prostate cancer therapeutics or multi-specifics, and groups using PSMA expression as a biomarker for patient selection, and treatment response in prostate cancer clinical trials. Invicro advances PSMA-PET standard of care by combining advanced image analytics and artificial intelligence with standard PET SUV readouts to ensure you are receiving the most meaningful data from your images. Invicro has overseen more clinical PSMA-PET scans (>3,600) than most imaging CROs and is one of the only organizations to support an FDA submission combining AI and quantitative PET.
Our full-service PSMA-PET solutions include:
- Complete global core lab support for Phase 0-III clinical trials
- Radiology expertise for study design, image analysis and sub-specialty reads (PCWG3)
- Advanced analytical tools for whole body image quantification to estimate disease burden, lesion localization and response to therapy
- Radiochemistry development and expert guidance on PSMA ligand access and utilization, including external manufacturing support
- Preclinical solutions for discovery research
- Extensive experience with Regulatory filing support (e.g., IND-enabling support)
Clinical Imaging Core Lab
Invicro supports early-phase, single-site and late phase, multi-site global clinical studies with a unique blend of scientific, medical, and operational practices. We are the leading imaging CRO in clinical PSMA-PET scans with over 3,600 managed. Invicro advances the PSMA-PET standard-of-care analysis and maximizes clinical data with our clinical solutions, including:
- First-in-human, single-site clinical trial support
- Study design and consultation
- Experience with eligibility and efficacy reads including VISION and RECIST 1.1/PCWG3
- Late stage, global multi-center clinical trial support with response criteria reads
- Advanced image analytics combining PET SUV readouts with AI
- Safety profile and therapeutic efficacy studies
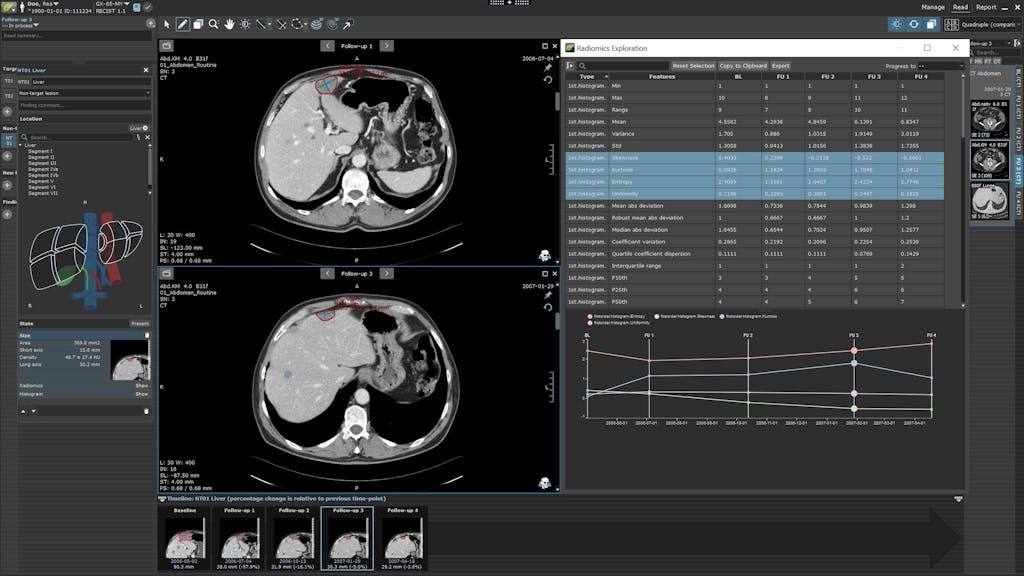
RECIST 1.1 Radiomics Exploration in MINT
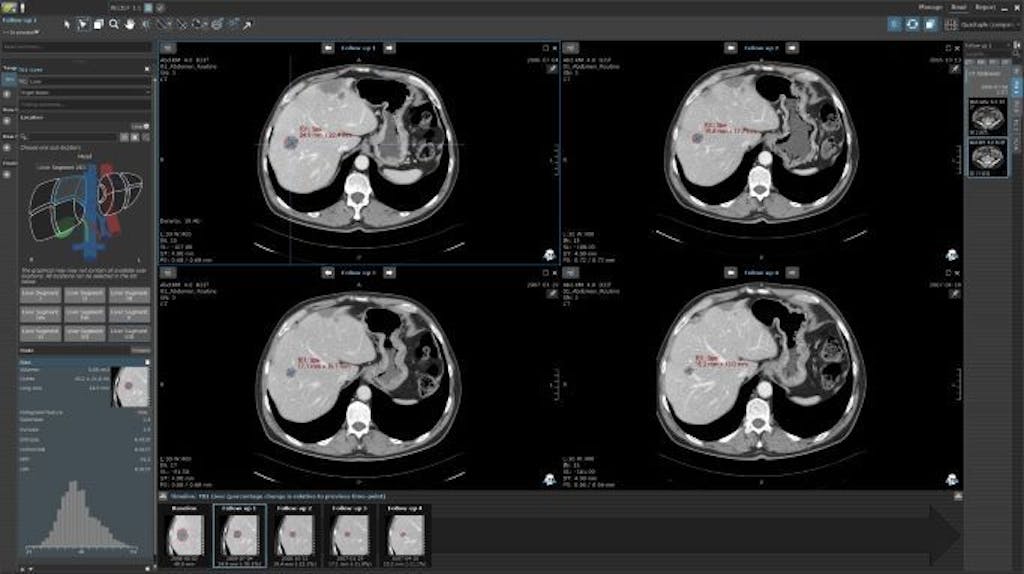
RECIST 1.1 Target Lesion Area Calculation in MINT
PSMA-PET Medical and Radiology Expertise
Invicro’s medical team is led by Dr. Phillip Kuo, an internationally recognized expert in PSMA-PET and is comprised of highly qualified readers and experienced MDs to help scale any project. Our offerings span from study design support to implementing read criteria such as PCWG3 for global clinical trials. Our existing and functional RECIST 1.1, PCWG3 and VISION-type reads are coded in MINT to allow for custom configuration to support your project goals. In addition, Invicro’s partners include multiple radiology groups that provide highly specialized reads to accommodate all project needs.
MINT Lesion Report – Response Evaluation

Image Analysis + Artificial Intelligence
Invicro’s advanced analytical tools specialize in whole body image quantification to estimate disease burden, lesion localization, and response to therapy. As one of the only imaging CROs to support an FDA submission using artificial intelligence (AI) and quantitative PET, our in-house team has experience supporting preclinical and clinical PSMA-PET imaging and therapeutic agent studies.
Our solutions span from customized and automated image processing techniques using deep learning to dosimetry for safety profile analysis. We build validated core lab workflows to meet the needs of your specific PSMA trials. Recently, Invicro and Telix Pharmaceuticals advanced a partnership to develop an AI platform to accompany Telix’s PSMA-PET imaging agent, Illuccix® – known as TelixAITM. TelixAITM seeks to increase the efficiency and reproducibility of clinicians’ imaging assessments using advanced analysis capabilities with an initial focus on prostate cancer.
Our advanced image analytic solutions include:
- Algorithms for automated and semi-automated lesion identification
- Image quality control and co-registration
- Preclinical dosimetry safety studies and clinical dosimetry analysis workflows
- Extrapolating dosimetry of therapeutics from imaging agents
- Expertise across various species

PSMA Radiochemistry Development and External Manufacturing
Invicro’s novel and commercial PSMA-PET ligand capabilities span from radiochemistry support for preclinical to first-in-human clinical studies and GMP production, utilizing our CMO partners.
Invicro works with the following isotopes:
- Lu177
- Ga68/ Lu177
- Ga68 labeled PSMA-617
- Ga68 labeled PSMA-I&T
- Ga68 labeled small molecules for PSMA targeting
- Additional Isotopes for PSMA imaging/therapy (In111, Lu177, Ac225, Cu64)
- 18F/18F-AlF4 chelation
- 11C
Preclinical PSMA-PET Solutions
Bringing new PSMA-PET imaging agents and therapeutics to market requires strong preclinical data. Invicro specializes in end-to-end preclinical solutions to drive discovery and streamline clinical translation of PSMA- targeted drugs.
Solutions include:
- Study design and consultation
- Establishment of radiolabeling methods followed by in vivo biodistribution and efficacy (PSMA radiotherapy studies using Ac225 and Lu177) in rodents
- Use of in vitro techniques (FACS analysis, radioligand binding assays and uptake, internalization, and retention assays) as methods of screening multiple compounds
- Use of imaging to assess tumor targeting, kinetics and biodistribution (Ga68 and 18F PET imaging, 177Lu SPECT imaging, Cut and Count biodistribution and mass dose analysis)
- Development of theranostic pairs to radiolabel PSMA small molecules for imaging and therapy of prostate cancer
- Validate and use [68Ga]PSMA-11 imaging to assess the therapeutic efficacy of a PSMA-targeted immuno-oncology agent in pre-clinical prostate tumor models (22Rv-1, LNCaP, PC3-PSMA and RM-1)
Theranostic development of a PSMA targeting small molecule for Prostate Cancer

Thought Leadership Content
Webinars
Segmentation of Lesions in Whole-Body PET/CT Using Deep Learning
Imaging Lessons from the VISION Trial
PSMA: Imaging Biomarker Development
The Changing Landscape of Response Assessment in Oncology
Imaging Biomarkers in Metastatic Prostate Cancer
Interviews
PSMA-PET Imaging Criteria Linked to Improved 177Lu-PSMA-617 Outcomes
68Ga PSMA Targeted PET Imaging in Prostate Cancer
Contact Us for More Information!
Sources
- https://www.cdc.gov/cancer/prostate/statistics/index.htm
- https://www.cdc.gov/cancer/prostate/basic_info/risk_factors.htm
- Zukotynski K and Kuo PH. 18F-DCFPyL PET/CT in men with prostate cancer. Radiology. 2022 Jul (online); doi.org/10.1148/radiol.221536
- https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-pluvicto-lutetium-lu-177-vipivotide-tetraxetan-treatment-adult