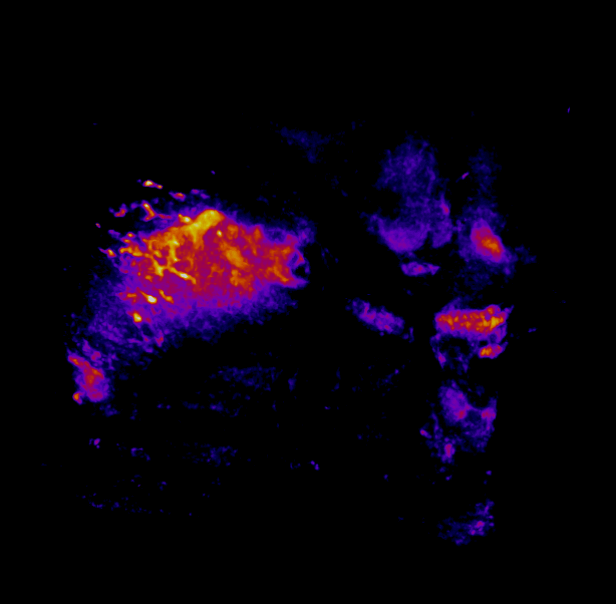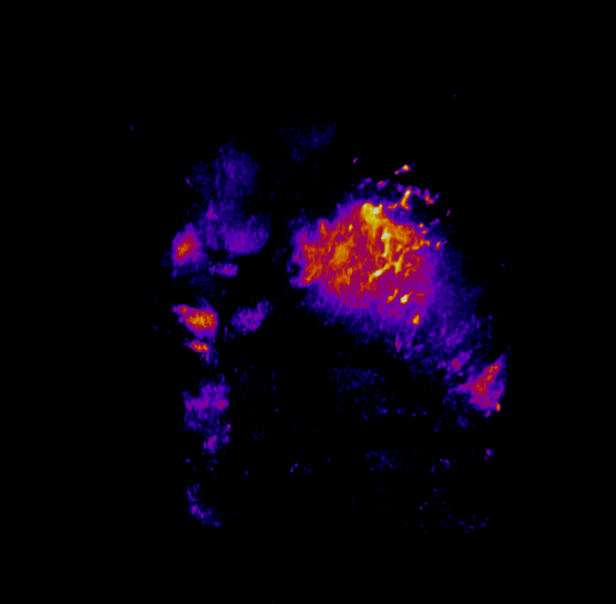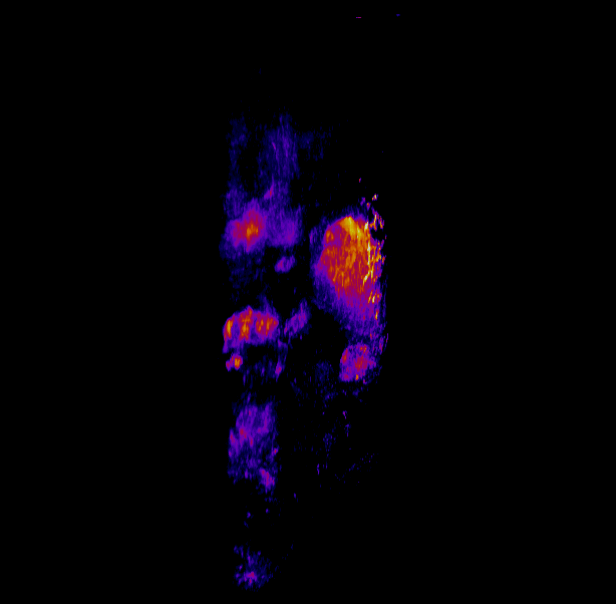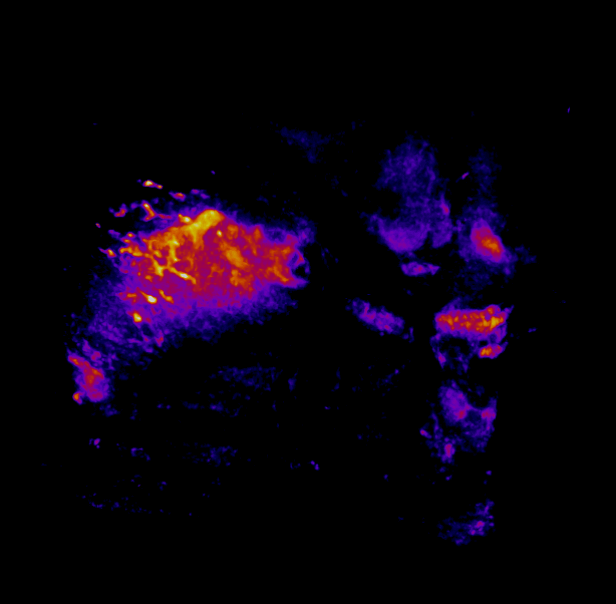Intraoperative tumor identification is prone to error because it is mainly based on visually identifying the changes in tissue morphology. To address this, surgical cameras are utilized to better distinguish tumors from normal tissue. A recent, first-in-human study showcased a novel technique using an FDA approved therapeutic antibody, Cetuximab, labeled with IRDey800 for more accurate tumor identification and resection (Rosenthal et al. 2015).
Resected tumors are subjected to pathological evaluation to determine the distribution of Cetuximab and its colocalization with epidermal growth factor receptor (EGFR). Although this approach provides high-resolution images of the antibody in the tumor tissue, it is constrained by randomized section collection and spatial resolution. Cryofluorescence Tomography (CFT), a novel ex vivo imaging technique developed at Invicro, can provide high-resolution fluorescence and bright field images to visualize the distribution of an antibody throughout an entire tumor sample.
In this study, CFT was utilized to visualize the distribution of Cetuximab-IRDye800 in a clinical squamous cell carcinoma sample (Figure 1). Following resection, the tumor was imaged using multiple near-infrared (NIR) imaging systems. The sample was then fixed in formalin and embedded in paraffin (FFEP) and subsequently sectioned and imaged with CFT. Block face white light and fluorescence images were simultaneously acquired for each section. The images were then reconstructed into a 3D volume to visualize the biodistribution of Cetuximab in the tumor. Maximum intensity projection (MIP) images show the antibody biodistribution throughout the processed tumor sample. When compared to the 2D near-infrared (NIR) images, the CFT images have a higher in-plane resolution and therefore, the surface and sub-surface level biodistribution can be observed in more detail.
Figure 1