
Amunolevulinic acid (ALA) is a metabolic precursor to protoporphyrin IX (PpIX). Should the metabolic pathway be overloaded with ALA, the fluorescent PpIX will be abundant in metabolically active regions such as malignant gliomas and meningioma, but generally increases fluorescence in epithelial and neoplastic tissue. One application of ALA is photodynamic therapy (PDT), a procedure that utilizes light, PpIX and oxygen. The light at a specific wavelength is used to excite PpIX which produces reactive oxygen radicals as byproducts. The oxygen radical interacts with cellular component and causes oxidative damage which results in cell death.
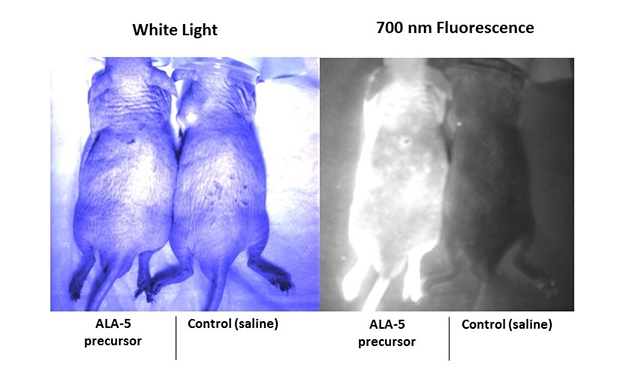
PDT is a growing technique for treating cancer. In addition, ALA is being used in fluorescence guided brain surgery for removing tumors. In this study, enhanced porphyrin autofluorescence resulting from ALA administration was assessed in mice using in vivo and ex vivo imaging techniques. A male hairless immunocompetent mouse was injected intravenously (IV) with 200 mg/kg of ALA. A control mouse was injected IV with 200 µL of saline. Both subjects were set aside for one hour to allow PpIX to accumulate in the body. The fluorescence was then imaged in vivo using a surgical fluorescence imaging system that is capable of dual channel imaging. The two imaging channels available on the instrument excite at 660 nm and 760 nm and collect at 700 nm and 800 nm, respectively. Although the PpIX fluorescence was visible in the 700 nm collection channel, the system was not capable of exciting PpIX because the absorption peak is at approximately 400 nm. Instead, an external UV LED (approximately 394-410 nm at peak intensity) was used to excite PpIX. To test the functionality of the UV LED and to demonstrate enhanced porphyrin fluorescence, in vivo dorsal epifluorescence images were collected using the 700 nm channel while using the UV LED to excite PpIX. The following white light and fluorescence images show enhanced porphyrin fluorescence in the skin of the test subject and not the control subject (Figure 1). However, due to the high autofluorescence of the skin and the relatively low penetration depth of optical light in tissue, we are unable to examine increased porphyrin fluorescence at depth.
Figure 1
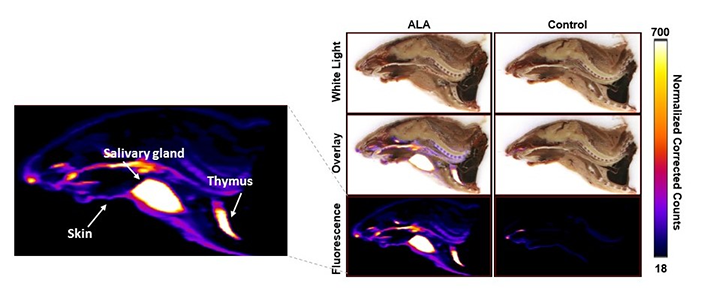
Cyrofluroescence tomography, a novel form of fluorescence imaging, was used to view the 3D distribution of porphyrin inside the body. At the one-hour time point, the subjects were sacrificed, frozen, and sliced with a slice thickness of 25 microns. Fluorescence and white light images were captured simultaneously. Image alignment was performed to construct a 3D fluorescence distribution. Finally, a correction algorithm was applied to the fluorescence images to mitigate the effects of optical absorption and scattering, providing a more accurate recovery of the fluorescence yield. A slice-by-slice overlay of the white light and the fluorescence images was generated in VivoQuant® for better visualization of the fluorescence distribution within the subject. A mid-sagittal slice of the reconstructed images is shown below (Figure 2).
Figure 2
In addition, 3D renderings of the subjects were created using maximum intensity projection for enhanced visualization of the fluorescence distribution and structure (Figure 3).
Figure 3
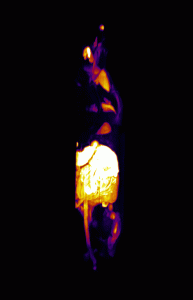
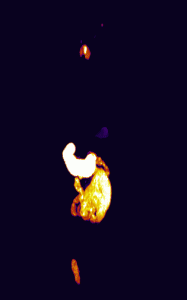
By using in vivo and ex vivo imaging techniques, an increase in porphyrin autofluorescence was shown in the skin, salivary glands, and the thymus of the subject injected with ALA. The use of Invicro’s software packages and imaging techniques have provided crucial information about the porphyrin fluorescence distribution that can be used to develop novel applications of ALA.
