
Radioligand Therapy & Theranostics Services
OVERVIEW
Radiopharmaceutical therapies represent an effective way to treat solid cancers by using tumor targeting small molecules, peptides or biologics to deliver a cytotoxic payload that induces DNA damage in tumor cells, while limiting damage to normal and healthy tissue.
Streamlined development, evaluation and clinical translation of radiopharmaceuticals requires a partner that has extensive scientific, regulatory and operational expertise. Working with a CRO that provides a complete solution ensures data is delivered in a timely manner to make a go/no-go decision on your imaging or
therapeutic candidates.
Services Include:
- Single-site, first-in-human through multi-site, late phase clinical study support
- Investigational New Drug (IND)/Clinical Trial Application (CTA) submission support with clinical protocol development
- Preclinical and clinical dosimetry and advanced quantitative image analysis
- Radiochemistry development – conjugation, radiolabeling, stability and immunoreactivity
- Preclinical proof-of-concept studies – biodistribution, efficacy, toxicology
- Good manufacturing practice (GMP) radiochemistry development and production support for imaging agents
Clinical Radiopharmaceutical Therapy Solutions
Seamless transition of your radiopharmaceutical into the clinic requires an imaging partner with experience supporting first-in-human, single-site and late phase, multi-site global clinical studies. Invicro has the unique blend of scientific expertise and operational experience to deliver on studies from Phase 0-III.
Clinical radiopharmaceutical therapeutic development services include:
- First-in-human, single-site clinical trial support
- Study design and consultation
- Criteria-based centralized independent reviews and internal analysis
- Late stage, global multi-center clinical trial support with response criteria reads
- Advanced image analytics including dosimetry
- Safety profile and therapeutic efficacy studies
Dosimetry and Advanced Image Analytics
Our in-house image analytics team has experience supporting preclinical and clinical radiopharmaceutical therapy studies, spanning from customized and automated image processing techniques using deep learning to dosimetry analysis for safety profile analysis.
Some advanced image analytic techniques include:
- Algorithms for automated and semi automated lesion identification
- Image quality control and co-registration
- Preclinical dosimetry safety studies and validated clinical workflows
- Extrapolating dosimetry of therapeutics from imaging agents
- Expertise across various species, radionuclides and novel administration routes
Three-Dimensional Dosimetry for Radiation Safety Estimates from Intrathecal Administration

Radiochemistry - Radioisotopes for Targeted Radiotherapy
Our team has experience with novel and commercially available radioisotopes used for targeted radiotherapy. Our capabilities for novel radiotracers span preclinical to first-in-human clinical studies and GMP production with Invicro’s CMO partners.
Invicro has experience with many isotopes including:
- 225Ac
- 177Lu
- 131I
- 111ln
- 68Ga/177Lu
- 111In/225Ac
- 203Pb/212Pb
- 67Cu/64Cu
Preclinical Radiopharmaceutical Therapy Solutions
Bringing new therapeutic radiopharmaceuticals to the market is a complex and challenging process. When filing an Investigational New Drug (IND) or Clinical Trial Application (CTA) prior to the initiation of human trials, it is crucial to have strong non-clinical data to support a new candidate.
Invicro provides complete preclinical support of your project, including:
-
- Study design and consultation
- Radiolabeling/conjugation to support alpha, beta, and gamma emitting isotopes
- in vitro/ex vivo cell and tissue-based assays
- Primary pharmacology studies to demonstrate MOA
- in vivo Imaging model development to support theranostic imaging
- Advanced image analytics
- Dosimetry
- IND support
- Development of chemistry, manufacturing and controls package for imaging agents
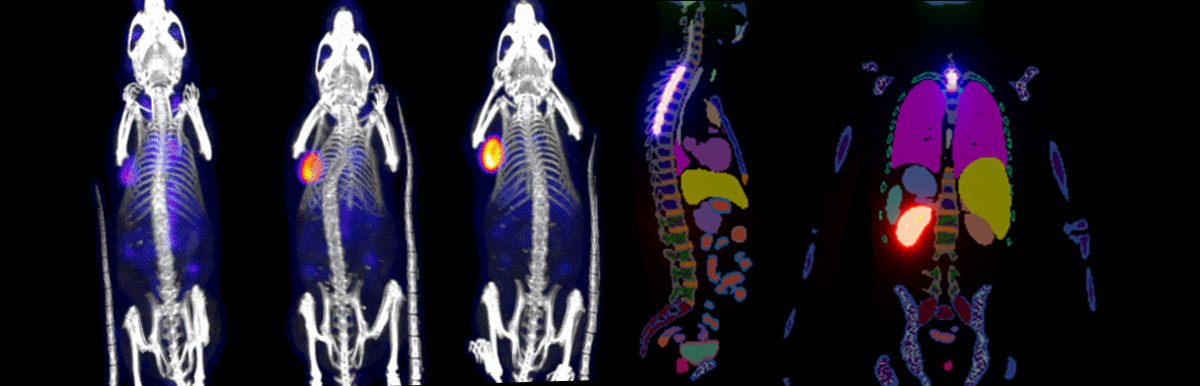
Antibody Imaging and Radionuclide Therapy – Imaging radiotherapy distribution in tumor positive mice

Thought Leadership Content
Webinars
The Resurgence of Theranostics: Challenges and Successes of Clinical Application
Translating Radiolabeled Biologics – Strategies for Successful IND Submission
PSMA: Imaging Biomarker Development
The Changing Landscape of Response Assessment in Oncology
Imaging Biomarkers in Metastatic Prostate Cancer
Interviews
PSMA-PET Imaging Criteria Linked to Improved 177Lu-PSMA-617 Outcomes
68Ga PSMA Targeted PET Imaging in Prostate Cancer
