
Clinical Imaging Core Lab
Clinical Trial Imaging CRO
Invicro is an medical imaging CRO with over 210 scientists, 60+ PhD’s and MD’s and 2100+ qualified imaging centers across the globe. We possess scientific and regulatory expertise, project management scale and operational excellence to manage all the clinical trial imaging components of your Phase I-IV clinical trials across the therapeutic spectrum and spanning imaging modalities . Our imaging core lab technology platforms support key decision-making in drug development by utilizing:
- Site training and data harmonization
- Patient screening and eligibility
- Criteria-based centralized independent reviews and internal analysis
- Confirmation of disease to ensure accurate patient management
- Late-stage, multi-center determination of efficacy
- Radiotracer supply chain and dose management capabilities
- Advanced medical image analysis pipelines
Learn more – Radiopharmaceutical therapy
Q&A Session – Obtaining High-Quality Imaging Data
Imaging Core Lab - Complete Project Support
Study Design
Deep scientific and medical expertise to support your study design
Study Start-Up
Investigator meeting, site training and qualification, and documentation creation/development
Image Mgmt.
Image submission and quality control via iPACS® – 21 CFR Part 11 Compliant
Read Mgmt.
Images are queued for subspeciality readers (blinded reads) – 21 CFR Part 11 Compliant
Image Analysis
Our team translates sophisticated, cutting-edge analytics into validated Core Lab workflows – 21 CFR Part 11 Compliant
Data Mgmt.
Data delivery via common formats including CDISC and study specific reporting
Learn how Invicro’s comprehensive operational and medical support helps to advance the imaging aspects of your clinical trials from start to finish.
Clinical Imaging Core Lab Expertise
Oncology
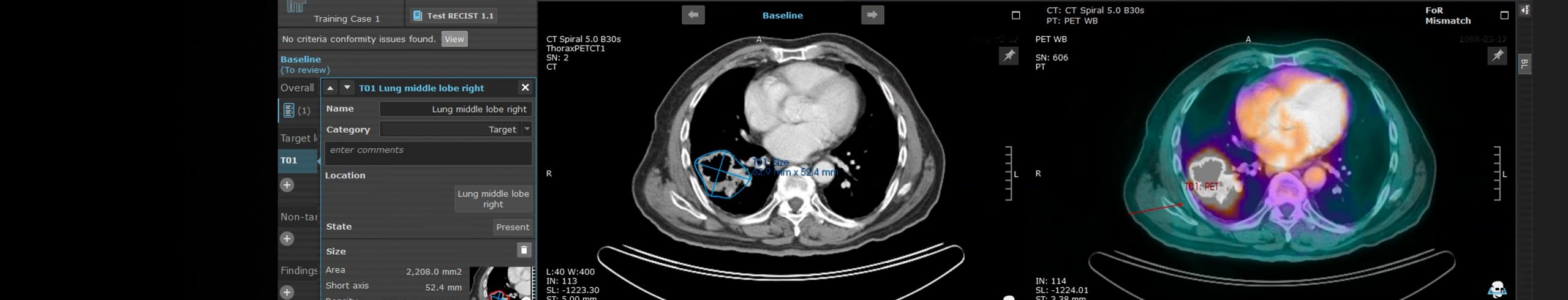
Invicro’s imaging core lab offers criteria-driven reads for standard-of-care projects and our state of the art in-house software enables 3D region-of-interest generation to support quantitative analysis methods, such as PERCIST, RECIST and PCWG. We also partner with subspecialist independent readers that focus on specific areas of expertise.
Validated Criteria Examples
-
- PERCIST
- RECIST 1.0/1.1
- PCWG 2.0/3.0
- RANO
- LUGANO
- IMWG
- CHESON
Modified for Immuno-oncology
-
- iRECIST
- irRECIST
- LYRIC
- irRC
Central Nervous System (CNS)
- 370+ clinical imaging trials managed
- >200 AD and PD Studies with >56,000 subjects imaged
- 90+ Phase I & II, multi-center trials conducted with PET, SPECT and MRI modalities
- 1500+ imaging centers qualified worldwide
- Two “in-house”clinical imaging centers with PET, SPECT and MRI capabilities
- Development and application of 90+ novel neuro imaging agents
- Redefining imaging analytic techniques
- Amyloid, Tau and Dat IQ Analytics
- SUV Generation
- Centiloid Mapping
Reader Programs
Invicro partners with leading institutions to select subspecialty radiologists, neuroradiologists and oncologists for our reader programs. We have a dedicated team that qualifies and selects readers to ensure that we have the most experienced professionals available to meet your study needs. All readers are trained according to your specific project requirements and performance is routinely monitored. Reads can be performed either as rolling reads as time points come in or batched and read as subjects come off study.
Different types of reads include:
- Eligibility
- 24-72 hour turnaround
- Confirmation of progression
- 24-72 hour turnaround
- Safety
- Double Read + Adjudication
- Double Read + Adjudication + Oncologist Clinical Read
- Consensus
- Majority
Medical Image Analysis
Invicro’s Medical Image Analysis team specializes in the evaluation of images to assess the performance of therapeutic and diagnostic agents. With more than 40,000,000 DICOM images analyzed, we have the expertise and scale to efficiently manage these processes to reduce variability and comply with regulatory requirements. At Invicro, image science meets image engineering. Our industry leading medical image analysis team translates sophisticated, cutting-edge analytics into validated Core Lab workflows, including:
Quantitative Oncology
The medical image analysis team supports standard analysis of FDG PET imaging, including PERCIST and other SUV-based metrics, metabolic tumor volume and total lesion glycolysis. The image analysis team also specializes in developing and applying a broad range of tools to support advanced quantitative oncology tracer characterization and analytics across a broad range of modalities and studies. Examples include:
- Powerful, multi-modal tumor and organ Segmentation tools
- A range of flexible Biomathematical Modeling tools
- Dosimetry calculations across radionuclide, administration route and species
- Integrated Radiomics pipelines using standard and custom feature vectors
- Preclinical and clinical support for Tracer Characterization
- Sophisticated batch Image Triage and preprocessing methods
For more information, check out some of our most popular case studies. A full list is also available in our case studies section!
Neurology
The image analysis team provides industry-leading tools for Neuro PET, SPECT and MRI analysis supporting quantification projects from discovery research through late phase trials. Invicro’s teams have analyzed thousands of clinical scans for Alzheimer’s Disease studies, utilizing validated SUVR pipelines, Centiloids and the state-of-the-art AmyloidIQ and TauIQ algorithms for Amyloid imaging and Tau imaging, respectively. Examples include:
- The novel IQ Analytics suite for Tau Imaging, Amyloid Imaging and DaT Imaging
- A range of flexible Biomathematical Modeling tools
- Validated pipelines across multiple quantitative outcomes
- Preclinical and clinical support for Tracer Characterization
- Sophisticated batch Image Triage and preprocessing methods
For more information, check out some of our most popular case studies. A full list is also available in our case studies section!
Imaging Modalities
Invicro’s imaging core lab is supported by iPACS®, our state-of-the-art image management platform. Utilizing a modality agnostic workflow to intake imaging data, we can QC, analyze and read a variety of imaging modalities, including:
PET/SPECT
Invicro is an industry leader in managing Phase I-IV clinical trials utilizing PET & SPECT imaging to support trial objectives. Our expertise includes:
- Imaging protocol development by Invicro scientists, physicians and industry key opinion leaders
- 1000+ PET qualified imaging centers in 35+ countries and 150+ SPECT qualified imaging centers in 15+ countries
- Phantom analysis algorithms for site instrument assessment and performance metrics
- Use of our internally developed iPACS software platform for fully web-based data submission, incorporating real-time conformity checks, configurable and automated study-specific workflows, and integrated query module
- Real-time image data review by modality experts for adherence to imaging guidelines and site-specific approved parameters for data standardization and harmonization
- Global network of expert radiologists and nuclear medicine physicians for study eligibility determination and drug efficacy assessments
- Automated pipelines for objective, quantitative analysis of radiotracer distribution and localization fully validated for compliance regulatory guidelines
MRI
Invicro has extensive expertise in conducting clinical MRI exploratory, disease modification and patient phenotyping/segregation studies.
- Three in-house, state-of-the-art systems for advanced MRI in our London clinic
- 475+ MRI qualified imaging sites in 25+ countries
- Centers are required to acquire ACR MRI phantom data using specific to characterize scanner performance
- Patient protocols are established and utilized for validation
- Our team of scientists are also actively engaged in trials with novel modalities and techniques such as resting-state fMRI, phMRI, MRS, DCE-MRI, ALS-MRI
Others
Invicro’s imaging core lab supports a variety of additional imaging modalities including:
- CT
- NM Bone Scan
- Echo
- X-ray, including Dynamic X-ray
- Photography
- Ultrasound
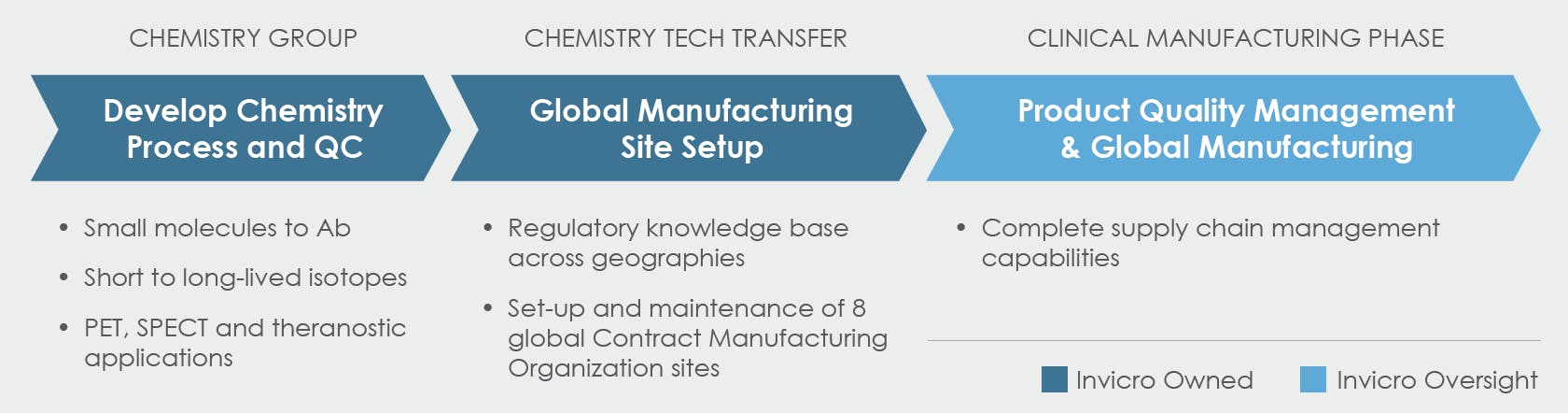
Manufacturing and Supply Chain Service
Therapeutic Areas
Thought Leadership Content
Case Studies
Rhesus Teeth Segmentation by Use of Multi-Atlas Library
Lipid Quantification Tool Development Using Multi-Echo MRI
Webinars
Segmentation of Lesions in Whole-Body PET/CT Using Deep Learning
Utilizing Imaging Agent Biomarkers for Improved Patient Management – Challenges and Successes
PSMA: Imaging Biomarker Development
The Changing Landscape of Response Assessment in Oncology
Molecular Imaging in Neurodegenerative Disorders: Developing a Translational Toolbox
Wherefore art Tau? Developing Methods for the Qualitative Interpretation of Tau PET
The IQ Analytics Platform: Amyloid-IQ, Tau-IQ and Dat-IQ
What Can we Learn from Primary Age-Related Tauopathy (PART)
Chemistry External Manufacturing and Network Management
Obtaining High Quality Imaging Data from Clinical Trials
White Papers
Q&A Session – Obtaining High-Quality Imaging Data
Infographics
Imaging Core Lab – Experience
Imaging Core Lab – Complete Project Support